Abstract
Introduction: Heparin-induced Thrombocytopenia (HIT) is a potentially life and limb threatening pro-thrombotic syndrome triggered by antibodies to platelet factor 4 (PF4)/polyanion complexes. Commonly used screening tests such as the PF4/Heparin ELISA (PF4 ELISA), while easy to perform in the near-patient setting, are highly non-specific. In contrast, more accurate tests such as the serotonin release assay (SRA) are technically complex, require radioactivity and are performed in only a handful of reference laboratories. These diagnostic challenges lead to both unnecessary anticoagulant use as well as avoidable cases of severe bleeding. Recent reports suggest that a relatively simple three-hour flow cytometry-based test that uses PF4-treated normal platelets, the PEA (PF4-dependent p-selectin Expression Assay), is at least as accurate as the SRA for identifying patients who have HIT (Thromb Haemost. 2015 Nov 25;114(6):1322-3; Chest. 2016 Sep;150(3):506-15; Chest. 2017 Oct;152(4):e77-e80). Here we describe results of a large prospective study designed to compare the accuracy of the SRA and PEA for HIT diagnosis in a clinical setting.
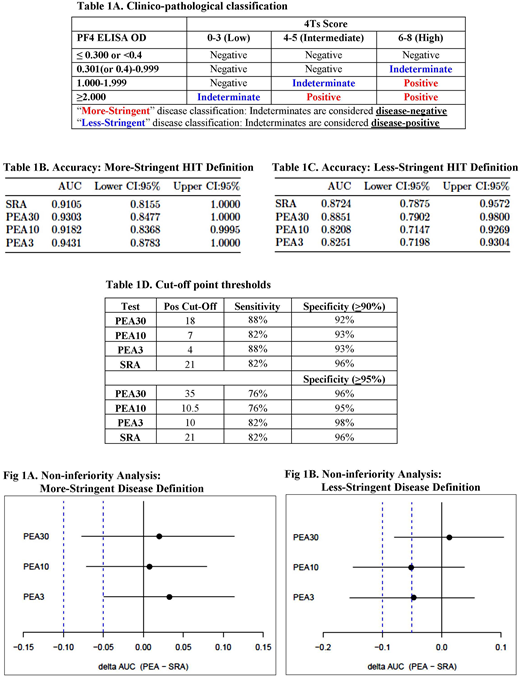
Methods: The PEA and SRA were performed on serum samples obtained from adult patients (≥18 yrs.) from 2 large tertiary care academic hospitals from May 2016 to Sep 2017. All inpatients for whom PF4 ELISA testing was ordered were included in the study. The PEA was performed with platelets pretreated with PF4 at 3 concentrations: 30mcg/mL (PEA30), 10mcg/mL (PEA10) and 3mcg/mL (PEA3). Sera were batch-tested in both assays (SRA and PEA) using the same target platelets. The central laboratory was blinded to clinical history and to the results of the PF4 ELISA performed by participating hospitals at the time of diagnosis. Both centers performed IgG-specific PF4 ELISAs and utilized manufacturer-recommended cut-offs for positivity. Predefined "more-stringent" and "less stringent" criteria based on clinical findings (4Ts score) and results of PF4 ELISA testing were used to define the likelihood of a patient having HIT (Table 1A). Receiver Operating Curve (ROC) analysis was performed and Area Under the Curve (AUC) of each assay was calculated to assess their diagnostic accuracy. Since high test specificity was desired, positive cut-offs that maximized the sum of sensitivity and specificity in a specificity range of ≥90% and ≥95% were derived. To evaluate non-inferiority of the PEA to the SRA, we assessed whether the lower limit of the one-sided 95% confidence interval of the ΔAUC (PEA-SRA) was higher than a non-inferiority margin of 5% and 10%.
Results: A total of 440 patient samples were tested with both assays. Seventeen and 28 patients were classified as having true HIT based on the use of the more- and less-stringent criteria, respectively. When classified by more-stringent criteria, the AUC was numerically greater for each of the three PEA tests than for the SRA (Table 1B). All tests had excellent accuracy (AUC>0.90, "excellent" by the Traditional academic point system). Using less-stringent criteria, the PEA30 had the highest AUC estimate followed by the SRA (Table 1C). Positive cut-offs generated at test specificities of ≥90% and ≥95% using data classified by more stringent criteria are shown in Table 1D. PEA3 was non-inferior to the SRA at a 5% non-inferiority margin, and all PEA tests were non-inferior at the 10% margin using the more stringent classification for true HIT (Fig 1A). In the less-stringently classified data set, the PEA30 was non-inferior to the SRA at a 10% margin (Fig 1B).
Conclusions: The PEA, a 3-hour, non-radioactive functional HIT assay demonstrated high diagnostic accuracy and was non-inferior to the SRA using commonly accepted non-inferiority margins for diagnostic testing (5-10%). The technical advantages of the PEA over the SRA may encourage its widespread adoption as a rapid-turnaround, accurate and near-patient diagnostic for patients with suspected HIT.
Jones:BloodCenter of Wisconsin: Patents & Royalties. Curtis:Ionis Pharmaceuticals: Consultancy. Bougie:BloodCenter of Wisconsin: Patents & Royalties. Aster:BloodCenter of Wisconsin: Patents & Royalties. Garcia:Shingoi: Consultancy; Retham Technologies LLC: Consultancy; Incyte: Research Funding; Daiichi Sankyo: Research Funding; Bristol Meyers Squibb: Consultancy; Portola: Research Funding; Janssen: Consultancy, Research Funding; Pfizer: Consultancy; Boehringer Ingelheim: Consultancy. Padmanabhan:BloodCenter of Wisconsin: Patents & Royalties: Patent/royalties for HIT diagnostic testing; Veralox Therapeutics: Other: Advisory Board; Retham Technologies LLC: Equity Ownership, Other: Retham seeks to develop HITDx(TM), a new assay for HIT diagnosis; TerumoBCT: Consultancy; GE Healthcare: Other: Material support for Clinical Quality Improvement Initiative; Janssen Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.